
The Aufbau principle is the first rule of ground-state electron configuration.Ground state electron configuration rules Aufbau principle Next, we need to find out in what orbitals does silicon places its electrons. However, a silicon atom has 14 protons, neutrons, and electrons in its neutral state. The ground state configuration of an atom Now, we know what the ground state is, and we have a brief idea about it. Now, the position of the electrons in our atom tells us lots of information about their atom structure.
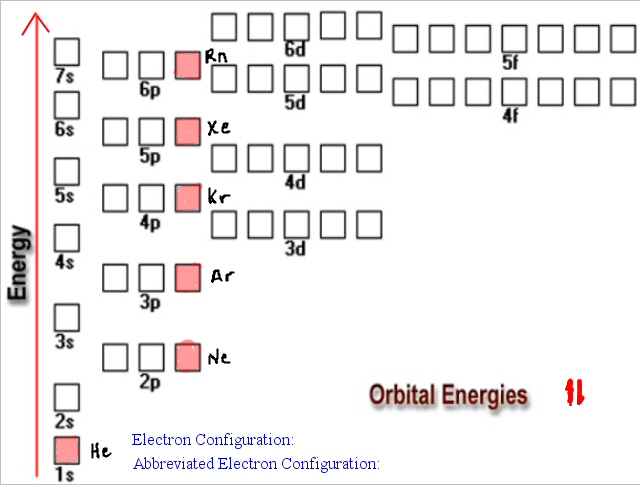
However, It is the easiest electron configuration to maintain. However, this configuration is ideal for the atom. It is the lowest level of energy we can have. Now, this is the reason why its name is the ground state. However, the ground-state electron configuration tells us the lowest possible energy configuration for an atom or a molecule. The ground-state electron configuration tells us which orbitals are going to be present in a given atom.

The ground-state electron configuration of Potassium.The ground-state electron configuration of Oxygen.The ground-state electron configuration of Nitrogen.The ground-state electron configuration of Sulfur.The ground-state electron configuration of Carbon.The ground-state electron configuration of elements.Ground state electron configuration rules.The ground state configuration of an atom.


 0 kommentar(er)
0 kommentar(er)
